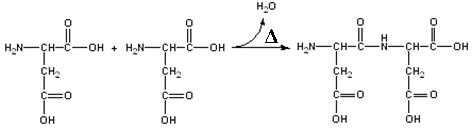
The polyamino acids and polysaccharides
are condensation polymers. This means that the chemical bonds that hold them together are formed by the loss of a molecule
of water between a reactive group of one building block and a reactive group of another building block. In the case
of the amino acids, a carboxylic
group (-COOH) of one amino acid condenses with an amine group (-NH2) of another amino acid to form an
amide bond.
|
|
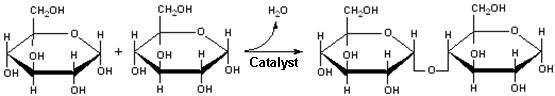
| Formation of maltose, a disaccharide |
In the case of the polysaccharides, each carbohydrate residue has several hydroxyl groups
(-OH) available for reaction. The hydroxyl of one residue condenses with a hydroxyl of another to form a C-O-C
bonded form. The general name for this is an ether linkage, but in the specific case of polysaccharides, the bonds
are termed glycosidic linkages.
|
|
| Vacuum and forced air ovens |
At Aquero Company, we start looking at potential reactions on a small scale, using vials
and dishes as reaction vessels. We mix the reactants in a proprietary blend and heat them at an appropriate temperature
for a proper amount of time. The products are characterized analytically, including assessments like Mw, yield, color, spectroscopic features, titration behavior, and so forth. Often,
one of the first tests is a lab-scale assessment of performance to see if the product might be useful.
|
|
| "Reaction vessels" for initial experiments |
|
|
| Thermal polycondensation reactor, 20 liter tank |
|
|
| Larger thermal (steam-jacketed, 250 to 500 gallons) mixer and reactor |
Once a product has been identified and optimized at the lab scale, often being produced only
in gram amounts, the next step is to produce enough of it to do more realistic testing. Kilograms of the product at
least are needed. This requires specialized equipment, some of which we have and maintain on-site, some of which we lease
as needed or otherwise access through partnerships.